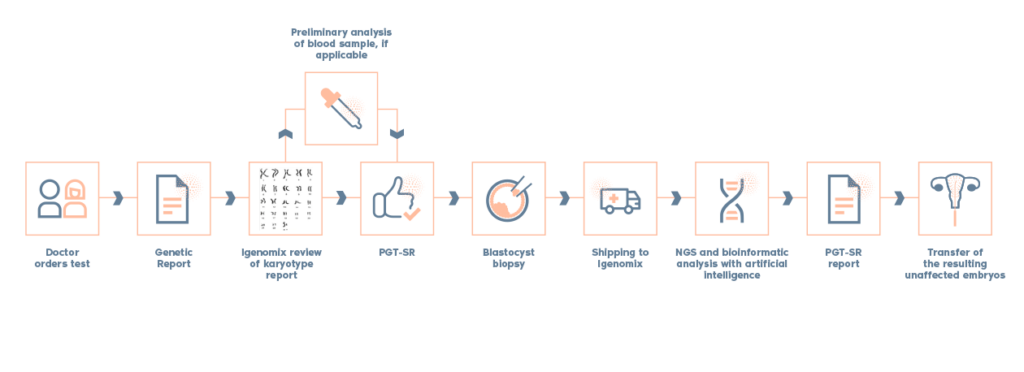
What is PGT-SR?
- The Preimplantation genetic testing for Structural Rearrangements (PGT-SR), is a test performed on embryo biopsies to screen embryos for chromosomal imbalances (extra or missing chromosome material) resulting from a parental structural rearrangement.
- In addition to screening for imbalances resulting from a parental structural rearrangement, PGT-SR also detects numerical chromosome abnormalities (aneuploidies). PGT-SR would be able to detect all of the same chromosome imbalances that would be assessed by PGT-A.
What is a Balanced Structural Rearrangement?
- A balanced structural rearrangement, such as translocation or inversion, involves a change in chromosome structure without gains or losses in chromosome material.
- A translocation is a type of chromosomal rearrangement that occurs when genetic material is exchanged between two chromosomes. An inversion occurs when a chromosome segment is flipped within the chromosome.
- Typically, a balanced structural rearrangement does not cause health concerns in carriers. However, a carrier of a balanced rearrangement has a higher risk of producing embryos with an unbalanced structural rearrangement (gain and/or loss of chromosome segments), which may lead to infertility, failed implantation, pregnancy loss, or the birth of a child with developmental delays and multiple congenital anomalies.
- Children who inherit the balanced structural rearrangement from their carrier parent are not expected to have health concerns.
- PGT-SR increases the probability of selecting a chromosomally normal (without the parental chromosomal rearrangement) or balanced (with the same parental chromosomal rearrangement) embryo for transfer, thus leading to improved assisted reproduction techniques (ART) outcomes.
PGT-SR can be used for different types of structural rearrangements including:
Balanced reciprocal translocation:
Occurs when segments from two chromosomes have been broken and exchanged. Each chromosome in the pair now has a segment of material from the other chromosome, in place of the original segment without gains or losses in genetic material.

Inversion:
Occurs when a segment within the chromosome has ‘flipped over,’ and is now in an inverted orientation.

Robertsonian translocation:
This is a specific type of translocation, which occurs between acrocentric chromosomes (i.e. chromosomes 13, 14, 15, 21, and 22). Instead of swapping segments, the long arm of the two chromosomes fuse together to form a single chromosome.